In these reactions there is never a change in oxidation state in other words the charges stay the same. AlOH3 Type of reaction.

Double Replacement Reaction Practice Problems Examples Youtube
Predicting and determining the products using the reactivity series.

. In this type of reaction we can find a hydrogen displacement and sometimes rarely occurring reactions involving oxygen displacement. Mgs 2HClaq MgCl₂aq H₂g Also Cu is above Ag. There are five types of chemical reactions and equations that represent them.
For the first few reactions the type of reaction is listed you should predict the products then balance. NaOH HClO4 NaClO4 H2O. MgO Type of.
Types of double replacement reaction in aqueous solution by kamran_9090. The reactions in which a single reactant is oxidized and reduced is known as Disproportionation reactions. Try balancing these chemical reactions.
Double displacement 3 3 Mg 1 Fe 2O3 2 Fe 3 MgO Type of reaction Lab. 1 3 NaBr 1 H3PO 4 1 Na 3PO 4 3 HBr Type of reaction. SINGLE REPLACEMENT REACTIONS DOUBLE REPLACEMENT REACTIONS 1.
One example of an acid-base. In chemistry chemical reactions are frequently written as an equation using chemical symbols. HA BOH BA H₂O.
Types of Reactions Worksheet Solutions Balance the following equations and indicate the type of reaction taking place. Definition and examples of double replacement reactions. Types of Reactions Worksheet Solutions.
Predicting and balancing neutralization and precipitation reactions. The pattern of a double displacement reaction is like this. Types of reactions - double and single displacement by jiselag.
P 4 3NaOH 3H 2 O 3NaH 2 PO 2 PH 3. If every ion is a spectator ion then there was no. This enables chemists to predict which combinations will undergo single displacement reactions.
Double displacement reactions take place mostly in aqueous solutions wherein the ions precipitate and exchange of ions takes place. In general for reactions in aqueous solution this involves adding H OH H 2 O and electrons to compensate for the. 1 3 NaBr 1 H3PO 4 1 Na 3PO 4 3 HBr Type of reaction.
Combination Reactions - In combination reactions two or more molecules are combined together chemically to form a new substance compound. Chap 2 ChemiCal ReaCtions amd equations page 7 To Purchase Hard Book of From Amazon Click Here. For example Mg is above H in the series.
A displacement reaction can be single or double. Balancing Chemical ReactionsEquations - Is RHSLHS true always. 1 The stuff before the arrow is referred to as the reactants or reagents and the stuff after the arrow is called the products 2 The number of atoms of each element is the same on both sides of the arrow.
2H2 O2 2H2O CU H2SO4CUSO4 H2 2MG O2 2MGO 2NA CL2 2NACL and so on. The significance of single and. Below are some examples of Chemical ReactionsEquations.
It is a chemical reaction in which one of the products is precipitated as an insoluble solid. If youre seeing this message it means were having trouble loading external resources on our website. 2 Na H 2 SO 4 Na 2 SO 4.
The reactants are displayed on the left side of the equation and the products are shown on the right with the separation of either a single or double arrow that signifies the direction of the reaction. Single Displacement Reactions aqueous ONLY metals. Test chemistry-1 by zhsimoy.
For example when we burn magnesium ribbon or magnesium it gives grey-black ash of magnesium oxide. I mean is RHSLHS always true for all Chemical. In a double displacement reaction two sets of exchanges.
Chemical reaction word equation by Airii. A single displacement reaction involves a product where one chemical compound from the reactant side exchanges to the product. In any case acid-base reactions are pretty much the same thing as double displacement reactions except that water is one of the things thats made.
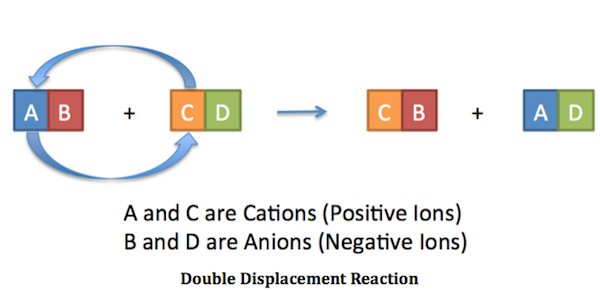
HBr Type of reaction. A s HX aq AX aq H 2g example. A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
Cu 2 AgNO 3 CuNO 3 2 2 Ag metal acid. Double displacement 3 3 Mg 1 Fe 2O3 2 Fe 3 MgO Type of reaction. Describing the overall electrochemical reaction for a redox process requires a balancing of the component half-reactions for oxidation and reduction.
Chapter 7 revision by Vino92TSA. Balancing and Classifying Chemical Reactions worksheet to classify and balance equations ID. EXAMPLES AgNO3 NaCl --- AgCl NaNO3 Silver nitrate sodium chloride silver chloride sodium nitrate PbNO32 CuSO4 --- PbSO4 CuNO32 Lead II nitrate copper.
Things to keep in mind when looking at the recipes for chemical reactions. If not the equation will probably never balance. Single displacement 4 1.
Correct balancing the equation is a simple task. Types of Reactions Worksheet Solutions Balance the following equations and indicate the type of reaction taking place. Balancing equation chemical reactions Jan 11 2022 1 akerkarprashant.
Double displacement reactions occur when a part of two ionic compounds are exchanged and make two new components. Aside from that its the same thing as a double displacement reaction. A redox reaction is the force behind an electrochemical cell like the Galvanic cell pictured.
A s BX aq AX aq B s example. Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation. Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents 638.
Even though there may be different numbers of molecules the number. Double displacement 2 3 CaOH 2 1 Al 2SO 43 3 CaSO 4 2 AlOH 3 Type of reaction. ZnCO3 heat ZnO CO2.
Predicting Products of Chemical Reactions This worksheet is designed to help you predict products of simple reactions of the four basic reaction types synthesis decomposition single replacement and double replacement and combustion reactions. The general form of a double-replacement also called double-displacement reaction is. Given the double displacement reaction between aqueous solutions of phosphoric acid and lead II acetate.
Another example is the reaction between magnesium and nitrogen. Cus 2AgNO₃aq. CeAB ceCD rightarrow ceAD ceCB In this reaction ceA and ceC are positively.
In this question you must recognize that perchlorate ClO4- and hydroxide OH- are polyatomic ions and will not break apart. When reactions have heat as a reactant it is very likely that they will involve. As you can see the H and B switched places which is where the water came from.
Also this is an acid-base reaction so the products should be salt and water. We predict that Mg should replace the H in HCl forming MgCl₂ and gaseous H₂. We predict that Cu should replace the Ag in AgNO₃ forming CuNO₃₂ and solid Ag.
For More Details Whatsapp at 8905629969 t pdf o v a i o The common types of double displacement reactions are as follows. Definition of single replacement or single displacement reactions. For example when a solution of barium chloride is mixed with.
Double displacement 2 3 CaOH 2 1 Al 2SO 43 3 CaSO 4 2 AlOH 3 Type of reaction. Balance the following equations and indicate the type of reaction taking place.
How Do You Balance Double Replacement Reactions Socratic

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Writing And Balancing Reactions Double Replacement Youtube

Double Replacement Double Displacement Reaction

Introduction To Double Replacement Reactions Youtube

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

What Is Double Replacement Reaction Example Share Education

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
0 comments
Post a Comment