Draw sketch Step 2. As we know lewiss.
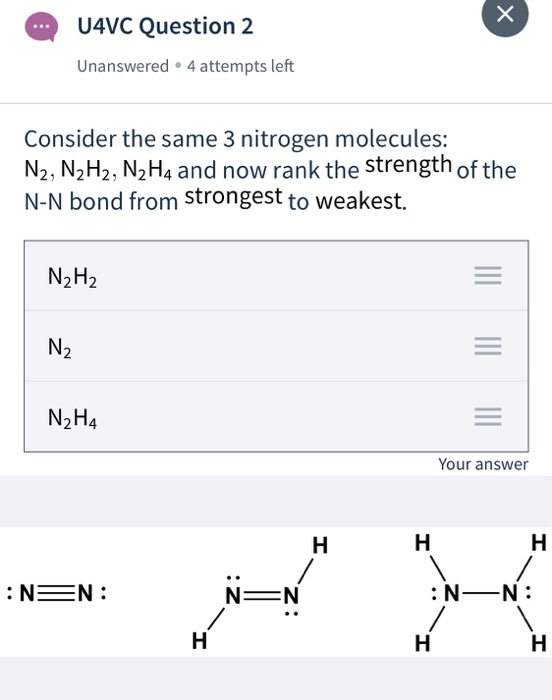
Solved H U4vc Question 1 Unanswered 4 Attempts Left Chegg Com
To summarize this blog post on N2H2 we can say the following.

. Welcome to my Channel. Draw the molecule by placing atoms on the grid and connecting them with bonds. There are 12 valence electrons for this molecule.
In the lewis structure of N2H2 there is a double bond between the two nitrogen. It is a yellowish-colored gas having both cis and trans isomers. Heres how you can draw the N 2 H 4 lewis structure step by step.
The dipole moment for the N2H4 molecule is 185 D. Mark lone pairs Step 3. In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
The molecular geometry for the N2H4 molecule is trigonal pyramidal and the electron geometry is tetrahedral. Draw the Lewis structure of N2H4 whose skeletal structure is H2NNH2. This compound is most commonly known as diazene or diimide.
I have a PhD degree in Engineering and have been teaching in various colleges and universities for several years. Drawing the Lewis Structure for N 2 H 4. Draw sketch Step 2.
Draw the Lewis structures of N2H4 N2H2 and N2. N2H2 Lewis Structure Molecular Geometry Hybridization and MO Diagram. The electron geometry for.
It can be prepared from the decarboxylation of azodicarboxylic acid NCOOH2. Dinitrogen dihydride has the chemical formula of N2H2. Mark lone pairs Step 3.
Minimize charges again if there are Lets break down each step in detail. Mark charges Step 4. Each Nitrogen atom forms a single bond with one Hydrogen atom and a double bond with the neighboring Nitrogen atom.
Diazene or Nitrogen azide has a trigonal pyramidal shape. Hope you understand the lewis structure geometry hybridization and polarity of N2H4. N2H2 diimide has two nitrogen atoms and two hydrogen atoms.
Follow some steps for drawing the Lewis dot structure of N2H4. Hydrogen H only needs two valence electrons to have a full outer shell. The hybridization of N2H4 is sp3.
A step-by-step explanation of how to draw the N2H4 Lewis Dot Structure HydrizineFor the N2H4 structure use the periodic table to find the total number of. Include all hydrogen atoms and nonbonding electrons. Draw Lewis structures for the following molecules.
A step-by-step explanation of how to draw the N2H2 Lewis Dot Structure Dinitrogen dihydrideFor the N2H2 structure use the periodic table to find the total. Minimize charges Step 5. As you see in the molecular shape of N2H4 on the left side nitrogen is attached to the two hydrogen atoms and both are below of plane of rotation and on the right side one hydrogen is above and one is below in the plane.
This problem has been solved.

Draw The Lewis Structures Of N2h4 N2h2 And N2 Draw The Molecules By Placing Atoms On The Grid And Brainly Com
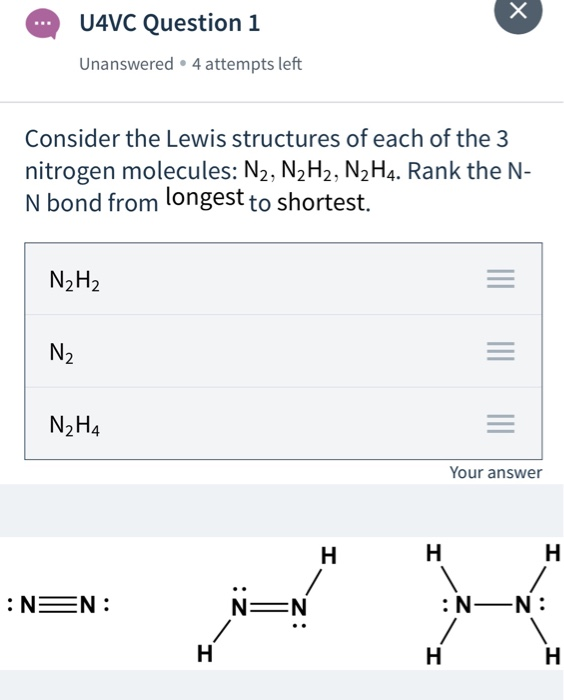
Solved H U4vc Question 1 Unanswered 4 Attempts Left Chegg Com

N2h4 Lewis Structure Geometry Hybridization And Polarity Techiescientist

N2h4 Lewis Structure Lewis Dot Structure For N2h4 Dinitrogen Tetrahydride Lewis Structure Youtube
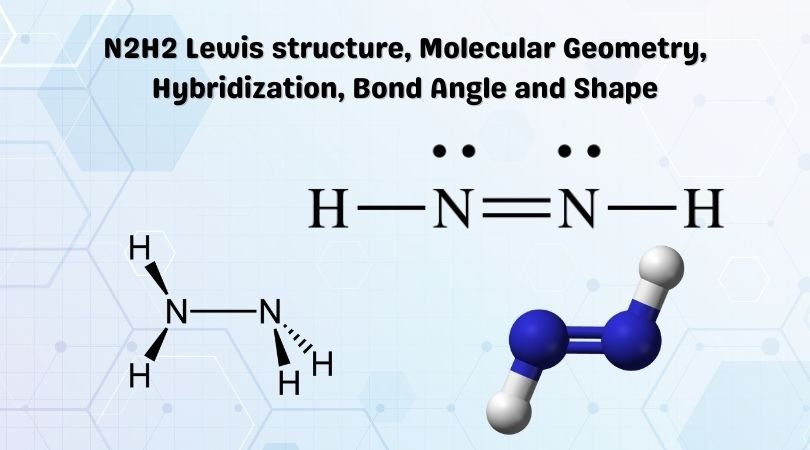
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape
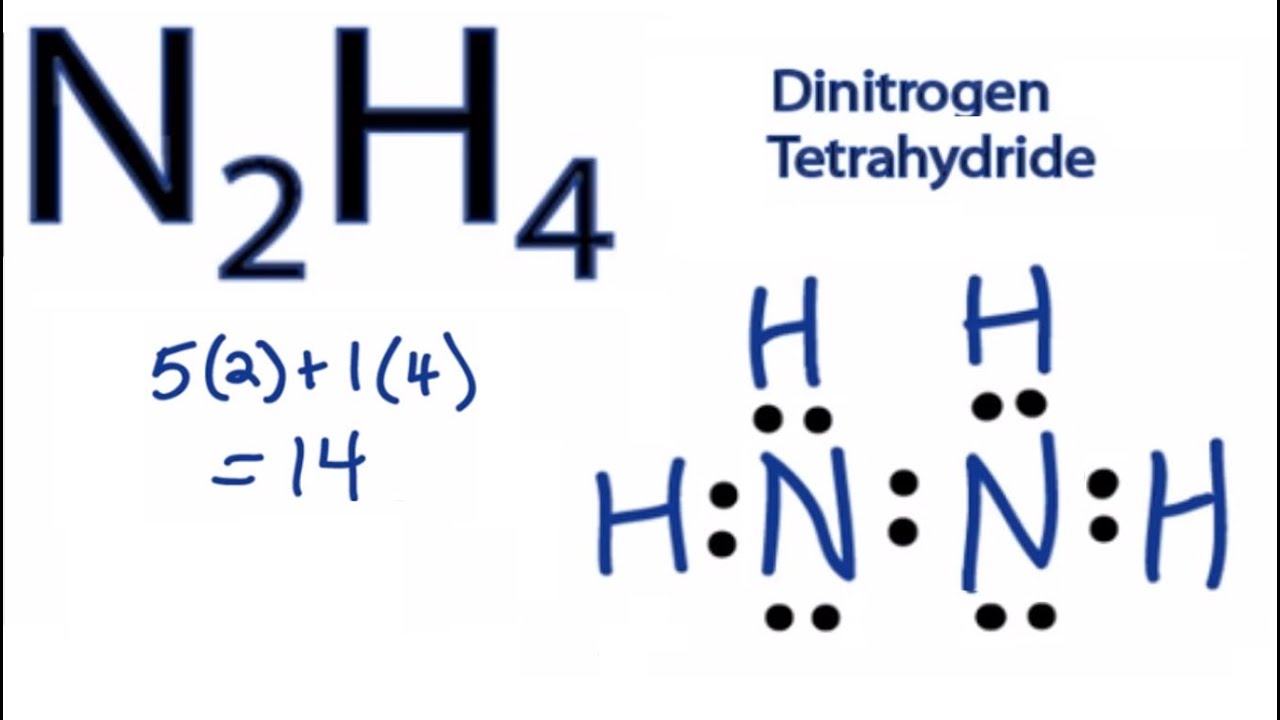
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
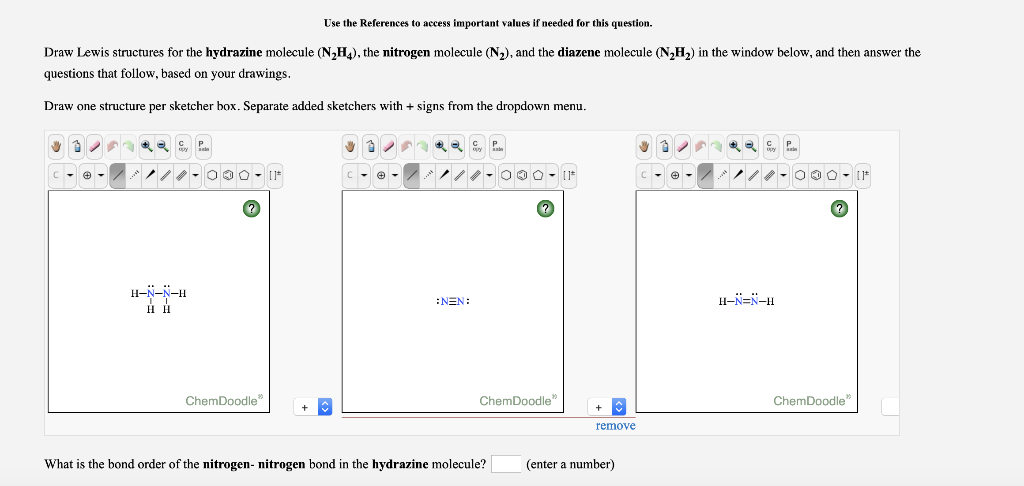
Solved Use The References To Access Important Values If Chegg Com

N2h4 Lewis Structure Molecular Geometry Polarity Hybridization Angle
0 comments
Post a Comment